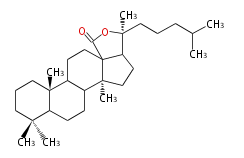Category:TP3
(→Cyclization) |
m (→Cyclization) |
||
| Line 93: | Line 93: | ||
| [[Image:Arrow00r.png]] | | [[Image:Arrow00r.png]] | ||
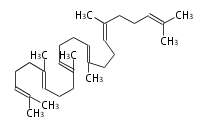
|colspan="2"|[[Image:2,3-oxidosqualene.png]] | |colspan="2"|[[Image:2,3-oxidosqualene.png]] | ||
| + | |- | ||
| + | | squalene-hopene [[Image:Arrow00d35.png]] cyclase | ||
| + | | | ||
| + | | lanosterol [[Image:Arrow00dl35.png]] synthase | ||
| + | | lupeol [[Image:Arrow00dr35.png]] synthase | ||
| + | |- | ||
| + | | deoxydammarenyl cation<br/>[[Image:Deoxydammarenyl cation.png]] | ||
| + | | | ||
| + | | protosteryl cation<br/>[[Image:Protosteryl cation.png]] | ||
| + | | dammarenyl cation<br/>[[Image:Dammarenyl cation.png]] | ||
|- | |- | ||
| [[Image:Arrow00d35.png]] | | [[Image:Arrow00d35.png]] | ||
| | | | ||
| − | | [[Image: | + | | [[Image:Arrow00d35.png]] |
| − | | [[Image: | + | | [[Image:Arrow00d35.png]] |
|- | |- | ||
| − | | [[Image: | + | |[[Image:Hopyl cation.png]] |
| | | | ||
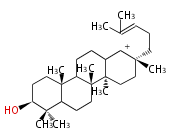
| − | | [[Image: | + | | lanosteryl cation<br/>[[Image:Lanosteryl cation.png]] |
| − | | [[Image: | + | | baccarenyl cation<br/>[[Image:Baccarenyl cation.png]] |
|- | |- | ||
| − | | [[Image: | + | | [[Image:Arrow00d35.png]] |
| | | | ||
| − | | [[Image: | + | | [[Image:Arrow00d35.png]] |
| − | | [[Image: | + | | [[Image:Arrow00d35.png]] |
|- | |- | ||
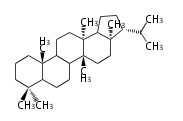
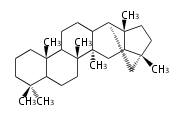
| − | | [[Image:Hopene.png]] | + | | hopene<br/>[[Image:Hopene.png]] |
| | | | ||
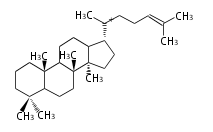
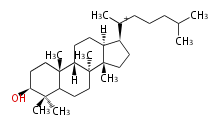
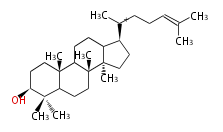
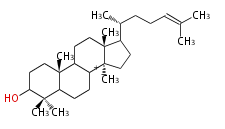
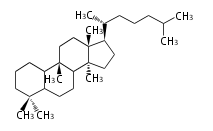
| − | | [[Image:Lanosterol.png]] | + | | lanosterol<br/>[[Image:Lanosterol.png]] |
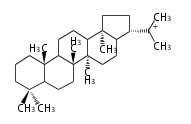
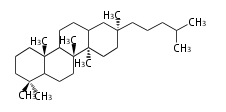
| − | | [[Image:Lupeol.png]] | + | | lupeol<br/>[[Image:Lupeol.png]] |
|} | |} | ||
Revision as of 15:39, 5 August 2010
Contents |
Triterpene (C30) Classes
Ring configuration
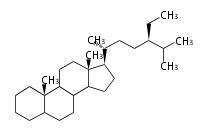
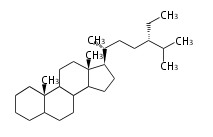
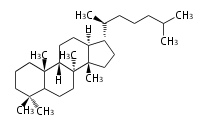
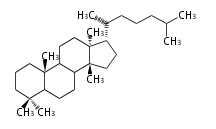
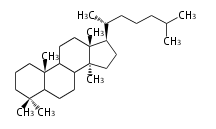
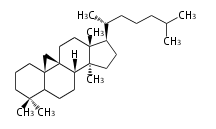
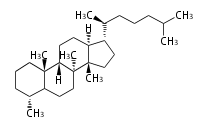
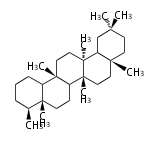
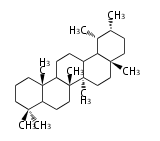
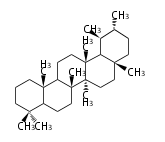
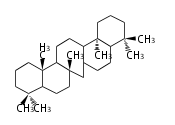
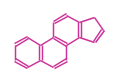
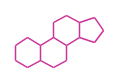
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. The rings B/C are always trans in all natural steroids. If the rings C/D are trans, it is called gonane. If its stereochemistry is unspecified, it is called sterane. Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are cis.
 |
|
| Cyclopenta[a]phenanthrene | Gonane |
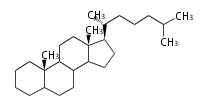
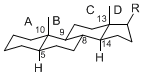
The majority of steroids have methyl groups sticking out from the bridgehead positions C-10 and C-13. When these methyl groups (or hydrogens) stand above the plane, they are called β-configuration. Those below the plane are called α-configuration. If the configuration at any site is unknown, it is indicated as ξ (Greek Xi). By default, hydrogen atoms or substituents at the positions C-8, 9, 10, 13, and 14 are assumed to be 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are.
 |
 |
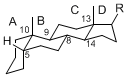
|
| cholestane backbone | 5α-configuration | 5β-configuration |
Biosynthesis
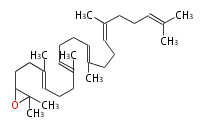
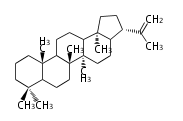
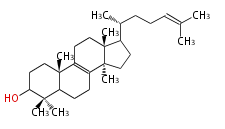
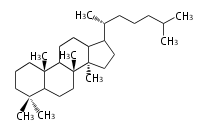
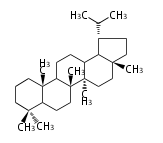
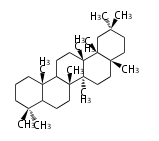
The starting point is squalene, which is formed by joining two FPPs tail-to-tail. In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes. In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol or cycloartenol by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts). Plant triterpenes arise from the dammarenyl cation. This molecule undergoes cyclization to form either the protosterol cation (through chair-boat cyclization) or the dammarenyl cation (through chair-chair cyclization). From the protosterol cation, lanosterol or cycloartenol occurs. From the 17β-dammarenyl cation, dammarane and other plant triterpenoids occur. From the 17α-damarenyl cation, hopanoids occur.
- animals, fungi, and yeast
- 2,3-oxidosqualene → lanosterol
- plants (including algae)
- 2,3-oxidosqualene → cycloartenol, dammarane
- bacteria
- 2,3-oxidosqualene → hopene
Cyclization
Design of Tri-terpene ID numbers ID番号の設計
<center> 12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
- y ... backbone structure (母核構造)
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
Cite error:
<ref> tags exist, but no <references/> tag was found
This category currently contains no pages or media.