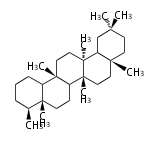
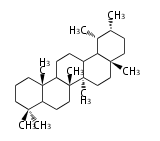
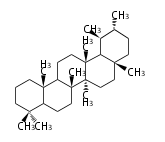
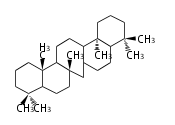
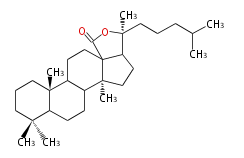Category:TP3
m (→Cyclization) |
(→Biosynthesis) |
||
| Line 51: | Line 51: | ||
{{Twocolumn| | {{Twocolumn| | ||
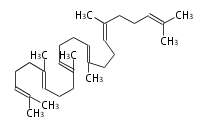
The starting point is squalene, which is formed by joining two FPPs tail-to-tail. | The starting point is squalene, which is formed by joining two FPPs tail-to-tail. | ||
| − | + | Bacterial cyclases use squalene directly, but those of the other species use | |
| − | + | 2,3-oxidosqualene for cyclization. | |
| − | + | ||
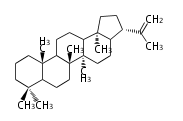
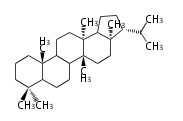
| − | + | * In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes. | |
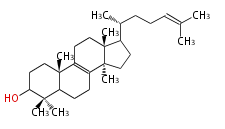
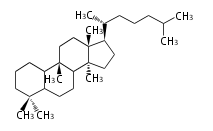
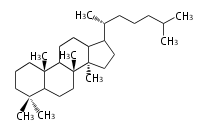
| − | + | * In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol or cycloartenol by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts). | |
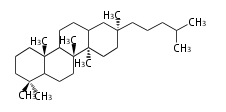
| − | + | * In plants, various triterpenes arise from the dammarenyl cation. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| | | | ||
| − | + | 始まりとなる物質はスクアレンで、二分子のファルネシル二リン酸が末尾どうしで結合して作られます。バクテリアの環化酵素はスクアレンをそのまま利用しますが、それ以外の生物種では2,3-オキシドスクアレンを利用します。 | |
| − | + | * バクテリアでは、スクアレンが環化して17α-ダンマラン型カチオンとなり、最終的にホペン他のトリテルペンになります。 | |
| + | * 真核生物では、2,3-オキシドスクアレンが環化してプロトステロール型カチオンとなり、ラノステロールやシクロアルテノールになります。 | ||
| + | * 更に植物の場合、ダンマラン型のカチオンを経て様々なトリテルペンが生成されます。 | ||
| + | }} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<center> | <center> | ||
{|style="text-align:center" | {|style="text-align:center" | ||
| + | |style="background-color:#ddf"| BACTERIA | ||
| + | | | ||
| + | |style="background-color:#fdd"| ANIMALS, FUNGI, and YEAST | ||
| + | |style="background-color:#dfd"| PLANTS | ||
| + | |- | ||
| squalene | | squalene | ||
| | | | ||
| Line 99: | Line 85: | ||
| lupeol [[Image:Arrow00dr35.png]] synthase | | lupeol [[Image:Arrow00dr35.png]] synthase | ||
|- | |- | ||
| − | | deoxydammarenyl cation<br/>[[Image:Deoxydammarenyl cation.png]] | + | | 17α-deoxydammarenyl cation<br/>[[Image:Deoxydammarenyl cation.png]] |
| | | | ||
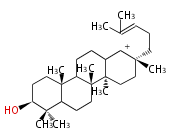
| protosteryl cation<br/>[[Image:Protosteryl cation.png]] | | protosteryl cation<br/>[[Image:Protosteryl cation.png]] | ||
| dammarenyl cation<br/>[[Image:Dammarenyl cation.png]] | | dammarenyl cation<br/>[[Image:Dammarenyl cation.png]] | ||
|- | |- | ||
| − | | [[Image:Arrow00d35.png]] | + | | D-ring [[Image:Arrow00d35.png]] expansion |
| | | | ||
| − | | [[Image:Arrow00d35.png]] | + | | Wagner-[[Image:Arrow00d35.png]] Meerwein shift |
| − | | [[Image:Arrow00d35.png]] | + | | D-ring [[Image:Arrow00d35.png]] expansion |
|- | |- | ||
|[[Image:Hopyl cation.png]] | |[[Image:Hopyl cation.png]] | ||
| Line 119: | Line 105: | ||
| [[Image:Arrow00d35.png]] | | [[Image:Arrow00d35.png]] | ||
|- | |- | ||
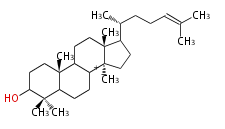
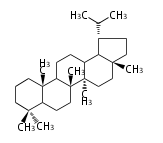
| − | | hopene<br/>[[Image:Hopene.png]] | + | |style="background-color:#ddf"| hopene<br/>[[Image:Hopene.png]] |
| | | | ||
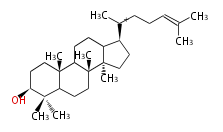
| − | | lanosterol<br/>[[Image:Lanosterol.png]] | + | |style="background-color:#fdd"| lanosterol<br/>[[Image:Lanosterol.png]] |
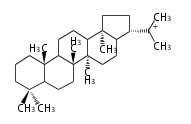
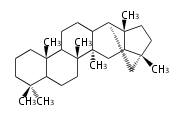
| − | | lupeol<br/>[[Image:Lupeol.png]] | + | |style="background-color:#dfd"| lupeol<br/>[[Image:Lupeol.png]] |
|} | |} | ||
| + | 2,3-オキシドスクアレンが環化する際に、ABC環が椅子-舟-5員環となる構造がまず生じ、椅子型C環へ拡張された後に5員型D環が生じたものがプロトステロールカチオン (椅子-舟-椅子-5員環) です。 | ||
| + | このカチオンからメチル基の転位反応を経て (Wagner-Meerweinシフト)、ラノステロールおよびシクロアルテノール (ともに椅子-椅子-椅子-5員環) が生じます。 | ||
| + | ダンマラン型の骨格は、2,3-オキシドスクアレンが環化する際に、椅子-椅子-椅子-5員環の構造がそのまま生じます。 | ||
| + | ホパノイドは複雑で2回の環拡張をおこないます。 | ||
<ref>Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T “Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis” Proc Natl Acad Sci USA 106(3):725-730, 2009</ref> | <ref>Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T “Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis” Proc Natl Acad Sci USA 106(3):725-730, 2009</ref> | ||
Revision as of 16:24, 5 August 2010
Contents |
Triterpene (C30) Classes
Ring configuration
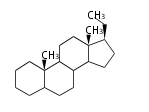
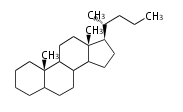
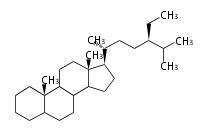
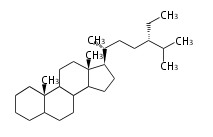
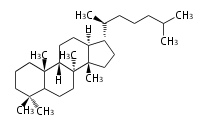
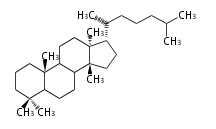
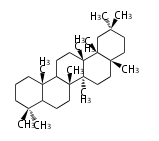
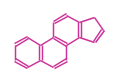
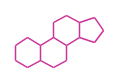
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. The rings B/C are always trans in all natural steroids. If the rings C/D are trans, it is called gonane. If its stereochemistry is unspecified, it is called sterane. Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are cis.
 |
|
| Cyclopenta[a]phenanthrene | Gonane |
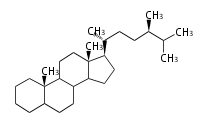
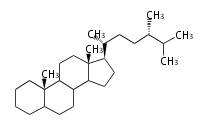
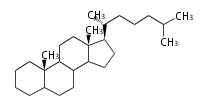
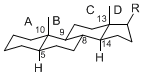
The majority of steroids have methyl groups sticking out from the bridgehead positions C-10 and C-13. When these methyl groups (or hydrogens) stand above the plane, they are called β-configuration. Those below the plane are called α-configuration. If the configuration at any site is unknown, it is indicated as ξ (Greek Xi). By default, hydrogen atoms or substituents at the positions C-8, 9, 10, 13, and 14 are assumed to be 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are.
 |
 |
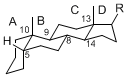
|
| cholestane backbone | 5α-configuration | 5β-configuration |
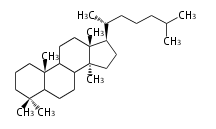
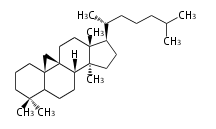
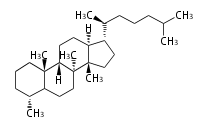
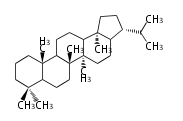
Biosynthesis
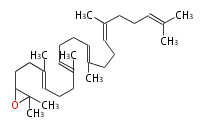
The starting point is squalene, which is formed by joining two FPPs tail-to-tail. Bacterial cyclases use squalene directly, but those of the other species use 2,3-oxidosqualene for cyclization.
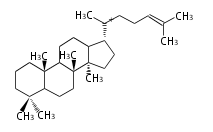
- In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes.
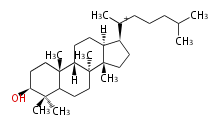
- In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol or cycloartenol by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts).
- In plants, various triterpenes arise from the dammarenyl cation.
2,3-オキシドスクアレンが環化する際に、ABC環が椅子-舟-5員環となる構造がまず生じ、椅子型C環へ拡張された後に5員型D環が生じたものがプロトステロールカチオン (椅子-舟-椅子-5員環) です。 このカチオンからメチル基の転位反応を経て (Wagner-Meerweinシフト)、ラノステロールおよびシクロアルテノール (ともに椅子-椅子-椅子-5員環) が生じます。 ダンマラン型の骨格は、2,3-オキシドスクアレンが環化する際に、椅子-椅子-椅子-5員環の構造がそのまま生じます。 ホパノイドは複雑で2回の環拡張をおこないます。 [1]
Design of Tri-terpene ID numbers ID番号の設計
<center> 12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
- y ... backbone structure (母核構造)
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
Cite error:
<ref> tags exist, but no <references/> tag was found
This category currently contains no pages or media.