Category:TP3
(→Biosynthesis) |
m (→Biosynthesis) |
||
| Line 55: | Line 55: | ||
* In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes. | * In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes. | ||
| − | * In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol or cycloartenol by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts). | + | * In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol (animals and fungi) or cycloartenol (plants) by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts). |
* In plants, various triterpenes arise from the dammarenyl cation. | * In plants, various triterpenes arise from the dammarenyl cation. | ||
| | | | ||
| Line 61: | Line 61: | ||
* バクテリアでは、スクアレンが環化して17α-ダンマラン型カチオンとなり、最終的にホペン他のトリテルペンになります。 | * バクテリアでは、スクアレンが環化して17α-ダンマラン型カチオンとなり、最終的にホペン他のトリテルペンになります。 | ||
| − | * 真核生物では、2,3- | + | * 真核生物では、2,3-オキシドスクアレンが環化してプロトステロール型カチオンを作り、プロトンとメチル基の転移 (Wagner-Meerweinシフト) を経て1,2-ラノステロール (動物や真菌類) やシクロアルテノール (植物) になります。 |
* 更に植物の場合、ダンマラン型のカチオンを経て様々なトリテルペンが生成されます。 | * 更に植物の場合、ダンマラン型のカチオンを経て様々なトリテルペンが生成されます。 | ||
}} | }} | ||
Revision as of 17:19, 5 August 2010
Contents |
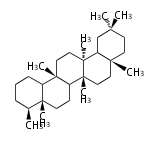
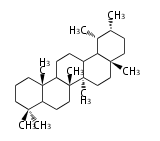
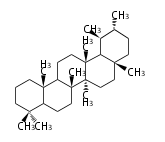
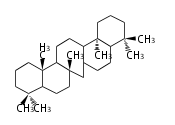
Triterpene (C30) Classes
Ring configuration
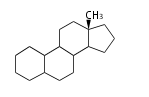
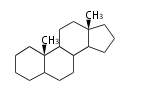
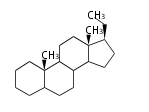
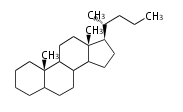
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. The rings B/C are always trans in all natural steroids. If the rings C/D are trans, it is called gonane. If its stereochemistry is unspecified, it is called sterane. Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are cis.
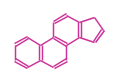 |
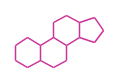
|
| Cyclopenta[a]phenanthrene | Gonane |
The majority of steroids have methyl groups sticking out from the bridgehead positions C-10 and C-13. When these methyl groups (or hydrogens) stand above the plane, they are called β-configuration. Those below the plane are called α-configuration. If the configuration at any site is unknown, it is indicated as ξ (Greek Xi). By default, hydrogen atoms or substituents at the positions C-8, 9, 10, 13, and 14 are assumed to be 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are.
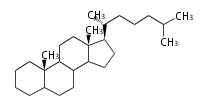 |
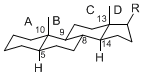 |
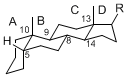
|
| cholestane backbone | 5α-configuration | 5β-configuration |
Biosynthesis
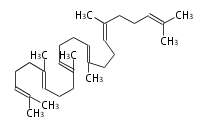
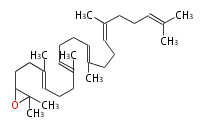
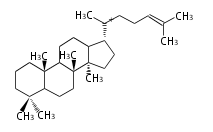
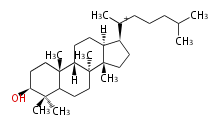
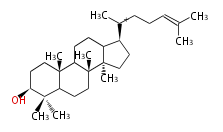
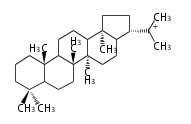
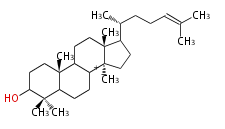
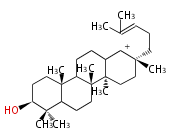
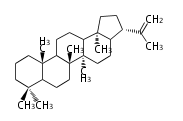
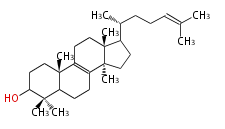
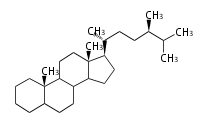
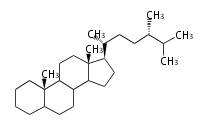
The starting point is squalene, which is formed by joining two FPPs tail-to-tail. Bacterial cyclases use squalene directly, but those of the other species use 2,3-oxidosqualene for cyclization.
- In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes.
- In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol (animals and fungi) or cycloartenol (plants) by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts).
- In plants, various triterpenes arise from the dammarenyl cation.
2,3-オキシドスクアレンが環化する際に、ABC環が椅子-舟-5員環となる構造がまず生じ、椅子型C環へ拡張された後に5員型D環が生じたものがプロトステロールカチオン (椅子-舟-椅子-5員環) です。 このカチオンからメチル基の転位反応を経て (Wagner-Meerweinシフト)、ラノステロールおよびシクロアルテノール (ともに椅子-椅子-椅子-5員環) が生じます。 ダンマラン型の骨格は、2,3-オキシドスクアレンが環化する際に、椅子-椅子-椅子-5員環の構造がそのまま生じます。 ホパノイドは複雑で2回の環拡張をおこないます。 [1]
Design of Tri-terpene ID numbers ID番号の設計
<center> 12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
- y ... backbone structure (母核構造)
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
Cite error:
<ref> tags exist, but no <references/> tag was found
This category currently contains no pages or media.