Category:TP3
m (→{{Bilingual|Oxidosqualene Cyclase in Eukaryotes|真核生物のオキシドスクアレン環化酵素}}) |
m (→{{Bilingual|バクテリア・シダ類のスクアレン環化酵素|Squalene Cyclase in Bacteria and Ferns}}) |
||
| (10 intermediate revisions by one user not shown) | |||
| Line 1: | Line 1: | ||
==Triterpene (C30)== | ==Triterpene (C30)== | ||
| − | =={{Bilingual|Ring configuration | + | =={{Bilingual|環の構造|Ring configuration}}== |
| − | ==={{Bilingual | + | ==={{Bilingual|ステロイド|Steroid}}=== |
{{Twocolumn| | {{Twocolumn| | ||
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. | The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. | ||
| Line 48: | Line 48: | ||
</center> | </center> | ||
| − | ==={{Bilingual | + | ==={{Bilingual|トリテルペン|Triterpenes}}=== |
{{Twocolumn| | {{Twocolumn| | ||
| Line 56: | Line 56: | ||
}} | }} | ||
| − | =={{Bilingual | + | =={{Bilingual|生合成|Biosynthesis}}== |
| − | ==={{Bilingual | + | ==={{Bilingual|概要|Overview}}=== |
{{Twocolumn| | {{Twocolumn| | ||
The starting point is squalene, which is formed by joining two FPPs tail-to-tail. | The starting point is squalene, which is formed by joining two FPPs tail-to-tail. | ||
| Line 76: | Line 76: | ||
{{Twocolumn| | {{Twocolumn| | ||
* In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes. | * In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes. | ||
| − | * In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol (animals and fungi), cycloartenol (plants) or parkeol (sea cucumbers) by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts). | + | * In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol (animals and fungi), cycloartenol (plants) or parkeol (sea cucumbers) by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts). |
| + | * In plants, a trace amount of phytosterols comes from lanosterol <ref>Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T (2009) Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis ''Proc Natl Acad Sci USA'' 106(3):725-730</ref> '''At3g45130''' is lanosterol synthase in ''Arabidopsis'' and its orthologs exist in asterids [[Species:Taraxacum|''Taraxacum officinale'']] and [[Species:Panax|''Panax ginseng]] and eurosid [[Species:Luffa|''Luffa cylindrica]]. Lanosterol synthase exists broadly among eudicots <ref>Kolesnikova MD, Xiong Q, Lodeiro S, Hua L, Matsuda SPT (2006) Lanosterol biosynthesis in plants ''Arch Biochem Biophys'' 447:87-95</ref>. Parkeol is also widespread in plants. | ||
* In plants, various triterpenes arise from the 17β-dammarenyl cation. | * In plants, various triterpenes arise from the 17β-dammarenyl cation. | ||
| | | | ||
* バクテリアでは、スクアレンが環化して17α-ダンマラン型カチオンとなり、最終的にホペン等のトリテルペンになります。 | * バクテリアでは、スクアレンが環化して17α-ダンマラン型カチオンとなり、最終的にホペン等のトリテルペンになります。 | ||
* 真核生物では、2,3-オキシドスクアレンが環化してプロトステロール型カチオンを作り、プロトンとメチル基の転移 (Wagner-Meerweinシフト) を経て1,2-ラノステロール (動物や真菌類)、シクロアルテノール (植物)や、パルケオール(ナマコ) になります。 | * 真核生物では、2,3-オキシドスクアレンが環化してプロトステロール型カチオンを作り、プロトンとメチル基の転移 (Wagner-Meerweinシフト) を経て1,2-ラノステロール (動物や真菌類)、シクロアルテノール (植物)や、パルケオール(ナマコ) になります。 | ||
| + | * 植物ではラノステロールからも一部の植物ステロールが合成されます。シロイヌナズナの '''At3g45130''' はラノステロール合成遺伝子であり、オーソログが菊類の [[Species:Taraxacum|''Taraxacum officinale'']] や [[Species:Panax|''Panax ginseng'']]、 真正バラ類の [[Species:Luffa|''Luffa cylindrica'']] にあることから、真正双子葉類に幅広く見られると考えられます。パーケオールも幅広い植物に見られます。 | ||
* 更に植物の場合、17β-ダンマラン型のカチオンから様々なトリテルペンが生成されます。 | * 更に植物の場合、17β-ダンマラン型のカチオンから様々なトリテルペンが生成されます。 | ||
}} | }} | ||
| − | ==={{Bilingual|Oxidosqualene Cyclase in Eukaryotes | + | ;References |
| + | <references/> | ||
| + | <br/> | ||
| + | |||
| + | ==={{Bilingual|真核生物のオキシドスクアレン環化酵素|Oxidosqualene Cyclase in Eukaryotes}}=== | ||
{{Twocolumn| | {{Twocolumn| | ||
Any path of reactions from the root (2,3-oxidosqualene) to any triterpene backbone with a colored background is catalyzed by a single enzyme called ''oxidosqualene cyclase'' (OSC) or ''terpene synthase h'' (tpsh).<ref>Terpene synthases a-f are responsible for mono-, sesquie- and diterpenes. Tps g is the squalene cyclase.</ref> | Any path of reactions from the root (2,3-oxidosqualene) to any triterpene backbone with a colored background is catalyzed by a single enzyme called ''oxidosqualene cyclase'' (OSC) or ''terpene synthase h'' (tpsh).<ref>Terpene synthases a-f are responsible for mono-, sesquie- and diterpenes. Tps g is the squalene cyclase.</ref> | ||
| Line 94: | Line 100: | ||
{| | {| | ||
|- | |- | ||
| − | ! {{Bilingual|Backbone Color Code | + | ! {{Bilingual|骨格色分け:|Backbone Color Code:}} |
! style="background-color:#fdd"| Animals, fungi, and yeast | ! style="background-color:#fdd"| Animals, fungi, and yeast | ||
! style="background-color:#dfd"| Plants only | ! style="background-color:#dfd"| Plants only | ||
! | ! | ||
|- | |- | ||
| − | ! {{Bilingual|Six-membered rings | + | ! {{Bilingual|6員環表記:|Six-membered rings:}} |
! colspan="2" | chair (C), or boat (B) | ! colspan="2" | chair (C), or boat (B) | ||
|} | |} | ||
| Line 114: | Line 120: | ||
|colspan="2" align="right"| | |colspan="2" align="right"| | ||
{| | {| | ||
| − | |lanosterol/<br/>cycloartenol<br/>syntase<ref>Lanosterol synthase is the most accessible enzyme among oxidosqualene cyclases, e.g. from mammalian liver or yeast. Cycloartenol synthase is the basic OSC in plants, although lanosterol synthase is also found. For the grouping of cyclases, check Xiong Q et al. (2005) in this page.<br/>'' | + | |lanosterol/<br/>cycloartenol<br/>syntase<ref>Lanosterol synthase is the most accessible enzyme among oxidosqualene cyclases, e.g. from mammalian liver or yeast. Cycloartenol synthase is the basic OSC in plants, although lanosterol synthase is also found. For the grouping of cyclases, check the review by Xiong Q et al. (2005) in this page.<br/>''References.'' |
* Corey EJ, Russey WE, Ortiz-de-Montellano PR (1966) 2,3-Oxidosqualene, an intermediate in the biological synthesis of sterols from squalene ''J Am Chem Soc'' 88:4750-1 | * Corey EJ, Russey WE, Ortiz-de-Montellano PR (1966) 2,3-Oxidosqualene, an intermediate in the biological synthesis of sterols from squalene ''J Am Chem Soc'' 88:4750-1 | ||
| − | * Kolesnikova MD, Xiong Q, Lodeiro S, Hua L, Matsuda SPT (2006) Lanosterol biosynthesis in plants ''Arch Biochem Biophys'' 447:87-95</ref> | + | * Kolesnikova MD, Xiong Q, Lodeiro S, Hua L, Matsuda SPT (2006) Lanosterol biosynthesis in plants ''Arch Biochem Biophys'' 447:87-95<br/> |
| + | In ''Arabidopsis'', cycloartenol synthase can be converted to lanosterol synthase with only two amino acid substitutions: His477 to Asn and Ile481 to Val. Tyr410 is also important for specificity. | ||
| + | * Lodeiro S, Schulz-Gasch T, Matsuda SPT (2005) Enzyme redesign: two mutations cooperate to convert cycloartenol synthase into an accurate lanosterol synthase ''J Am Chem Soc'' 127:14132-14133 | ||
| + | </ref> | ||
|[[Image:Arrow00dl35.png]] | |[[Image:Arrow00dl35.png]] | ||
|stepwise<br/>cyclization<ref>The cyclization process is stepwise, not concerted as previously thought. As one clue, squalene is not fully folded in the cyclase active site.<br/>''Ref.'' Reinert DJ, Balliano G, Schulz GE (2004) Conversion of squalene to the pentacarbocyclic hopene. ''Chem Biol'' 11:121-6</ref> | |stepwise<br/>cyclization<ref>The cyclization process is stepwise, not concerted as previously thought. As one clue, squalene is not fully folded in the cyclase active site.<br/>''Ref.'' Reinert DJ, Balliano G, Schulz GE (2004) Conversion of squalene to the pentacarbocyclic hopene. ''Chem Biol'' 11:121-6</ref> | ||
| Line 123: | Line 132: | ||
|align="left"| | |align="left"| | ||
{| | {| | ||
| − | |lupeol<br/>synthase | + | |lupeol/<br/>β-amyrin<br/>synthase<ref>Lupeol synthases are found in [[Species:Glycyrrhiza|''Glycyrrhiza glabra'']], [[Species:Betula|''Betula'' platyphylla]], [[Species:Taraxacum|''Taraxacum officinale'']], and [[Species:Olea|''Olea europea'']]. They form a clade (74-81% identical) that is distinct from other OSCs. Lupeol synthase evolved before the divergence of asterids and eurosids. On the other hand, β-amyrin synthases are considerably more distant (48-50% identical) than are the CAS enzymes (70-79% identical). β-amyrin synthases in eudicotsa nd monocots may have independent origins.<ul><li>Haralampidis K, Bryan G, Qi X, Papadopoulou K, Bakht S, Melton R, Osbourn A (2001) A new class of oxidosqualene cyclases directs synthesis of antimicrobial phytoprotectants in monocots ''Proc Natl Acad Sci U S A'' 98(23):13431-6 <li>Qi X, Bakht S, Leggett M, Maxwell C, Melton R, Osbourn A (2004) A gene cluster for secondary metabolism in oat: implications for the evolution of metabolic diversity in plants ''Proc Natl Acad Sci U S A'' 101(21):8233-8</ul></ref> |
|[[Image:Arrow00dr35.png]] | |[[Image:Arrow00dr35.png]] | ||
|stepwise<br/>cyclization | |stepwise<br/>cyclization | ||
|} | |} | ||
|- | |- | ||
| − | | 17β-protosteryl cation <small>(C-B-C)</small><ref>The C-17 chain of rotosteryl cation is β-configuration, not α.<br/> ''Ref.'' Corey EJ, Virgil SC (1991) An experimental demonstration of the stereochemistry of enzymic cyclization of 2,3-oxidosqualene to the protosterol system, forerunner of lanosterol and cholesterol. [http://pubs.acs.org/doi/abs/10.1021/ja00010a073 ''J Am Chem Soc'' 113:4025-6]</ref> <br/>[[Image:Protosteryl cation.png]] | + | | 17β-protosteryl cation <small>(C-B-C)</small><ref>The C-17 chain of rotosteryl cation is β-configuration, not α.<br/> ''Ref.'' Corey EJ, Virgil SC (1991) An experimental demonstration of the stereochemistry of enzymic cyclization of 2,3-oxidosqualene to the protosterol system, forerunner of lanosterol and cholesterol. [http://pubs.acs.org/doi/abs/10.1021/ja00010a073 ''J Am Chem Soc'' 113:4025-6]</ref> <br/>[[Image:Protosteryl cation.png|150px]] |
| 1,2-shift<br/>[[Image:Arrow00r.png]] | | 1,2-shift<br/>[[Image:Arrow00r.png]] | ||
| − | | colspan="2"|lanosteryl cation <small>(C- | + | | colspan="2" align="left"|lanosteryl cation <small>(C-B-C)</small><br/>[[Image:Lanosteryl cation.png|150px]] |
| − | | 17β-dammarenyl cation <small>(C-C-C)</small><ref>The C-17 chain of dammarenyl cation is β-configuration.<br/> ''Ref.'' Xiong Q, Rocco F, Wilson WK, Xu R, Ceruti M, Matsuda SPT (2005) Structure and reactivity of the dammarenyl cation: configuration transmission in triterpene synthesis. ''J Org. Chem. 70:5362-75</ref> <br/>[[Image:Dammarenyl cation.png]] | + | | 17β-dammarenyl cation <small>(C-C-C)</small><ref>The C-17 chain of dammarenyl cation is β-configuration.<br/> ''Ref.'' Xiong Q, Rocco F, Wilson WK, Xu R, Ceruti M, Matsuda SPT (2005) Structure and reactivity of the dammarenyl cation: configuration transmission in triterpene synthesis. ''J Org. Chem. 70:5362-75</ref> <br/>[[Image:Dammarenyl cation.png|150px]] |
|- | |- | ||
|colspan="2"| | |colspan="2"| | ||
| Line 164: | Line 173: | ||
|} | |} | ||
|- valign="top" | |- valign="top" | ||
| − | |colspan="2"| cation with the chain at C18 or C17 position<br/>[[Image:CBCC cation.png]]or [[Image:CBCC cation2.png]] | + | |colspan="2"| cation with the chain at C18 or C17 position<br/>[[Image:CBCC cation.png|150px]]or [[Image:CBCC cation2.png|100px]] |
| | | | ||
{| | {| | ||
|style="background-color:#fdd"| all steroids<br/>lanostane<br/>cycloartane<br/>cucurbitane<br/>ergostane etc. | |style="background-color:#fdd"| all steroids<br/>lanostane<br/>cycloartane<br/>cucurbitane<br/>ergostane etc. | ||
|} | |} | ||
| − | | | + | |style="vertical-align:bottom"| |
| − | {| | + | {| style="margin-left:auto;margin-right:0;" |
| − | + | ||
| − | + | ||
|style="background-color:#dfd"| baccharane<br/>shionane | |style="background-color:#dfd"| baccharane<br/>shionane | ||
| [[Image:Arrow00l35.png]] | | [[Image:Arrow00l35.png]] | ||
|} | |} | ||
| − | | baccarenyl cation <small>(C-C-C-C)</small><br/>[[Image:Baccarenyl cation.png]] | + | | baccarenyl cation <small>(C-C-C-C)</small><br/>[[Image:Baccarenyl cation.png|150px]] |
|- | |- | ||
| | | | ||
| Line 190: | Line 197: | ||
|} | |} | ||
| | | | ||
| − | | | + | | |
| − | + | {| style="margin-left:auto;margin-right:0;" | |
| E-ring cyclization<br/>(from 17α/β) | | E-ring cyclization<br/>(from 17α/β) | ||
| [[Image:Arrow00dl.png]] | | [[Image:Arrow00dl.png]] | ||
| Line 201: | Line 208: | ||
|} | |} | ||
|- valign="top" | |- valign="top" | ||
| − | | arborinyl cation <small>(C-B-C-C)</small><br/>[[Image:Arborinyl cation.png]] | + | | arborinyl cation <small>(C-B-C-C)</small><br/>[[Image:Arborinyl cation.png|150px]] |
| − | | colspan="2" align="left"| unnamed cation <small>(C-B-C-C)</small><br/>[[Image:Hanco cation.png]] | + | | colspan="2" align="left"| unnamed cation <small>(C-B-C-C)</small><br/>[[Image:Hanco cation.png|150px]] |
| − | | 21α-hopyl cation <small>(C-C-C-C)</small><br/>21β-moretyl cation <small>(C-C-C-C)</small><ref>The 21''R'' stereocenter is usually lost in hydride shift.</ref><br/>[[Image:Hopyl cation.png]] | + | | 21α-hopyl cation <small>(C-C-C-C)</small><br/>21β-moretyl cation <small>(C-C-C-C)</small><ref>The 21''R'' stereocenter is usually lost in hydride shift.</ref><br/>[[Image:Hopyl cation.png|150px]] |
| − | | H18α-lupyl cation <small>(C-C-C-C)</small><br/>H18β-lupyl cation <small>(C-C-C-B)</small><br/>[[Image:Lupyl cation.png]] | + | | H18α-lupyl cation <small>(C-C-C-C)</small><br/>H18β-lupyl cation <small>(C-C-C-B)</small><br/>[[Image:Lupyl cation.png|150px]] |
|- | |- | ||
|align="left"| | |align="left"| | ||
| Line 236: | Line 243: | ||
| | | | ||
|style="background-color:#dfd" align="left"| hopane (C-C-C-C)<br/> gammacerane (C-C-C-C-C)<br/> fernane (C-C-C-C)<br/> swertane (C-C-C-C-C)<br/> | |style="background-color:#dfd" align="left"| hopane (C-C-C-C)<br/> gammacerane (C-C-C-C-C)<br/> fernane (C-C-C-C)<br/> swertane (C-C-C-C-C)<br/> | ||
| − | |style="background-color:#dfd" align="left"| oleanane (C-C-C-C-C)<br/> lupane (C-C-C-C)<br/> germanicane<br/> taraxastane (C-C-C-C-C)<br/> ursane (C-C-C-C-C/B)<br/> friedomadeirane (C-C-C-C)<ref>E-ring cyclization precedes D-ring expansion.</ref><br/> | + | |style="background-color:#dfd" align="left"| oleanane<ref>β-amyrin has the oleanane skeleton</ref> (C-C-C-C-C)<br/> lupane (C-C-C-C)<br/> germanicane<br/> taraxastane (C-C-C-C-C)<br/> ursane (C-C-C-C-C/B)<br/> friedomadeirane (C-C-C-C)<ref>E-ring cyclization precedes D-ring expansion.</ref><br/> |
|} | |} | ||
</center> | </center> | ||
| − | <references/> | + | {| class="wikitable collapsible" style="width:100%" |
| − | + | ! References | |
| − | + | |- | |
| − | Reviews | + | | <references/> |
| + | |- | ||
| + | ! Reviews | ||
| + | |- | ||
| + | | | ||
* Xu R, Fazio GC, Matsuda SPT (2004) On the origins of triterpenoid skeletal diversity. ''Phytochemstry'' 65:261-291 PMID 14751299 | * Xu R, Fazio GC, Matsuda SPT (2004) On the origins of triterpenoid skeletal diversity. ''Phytochemstry'' 65:261-291 PMID 14751299 | ||
* Philips DR, Rasbery JM, Bartel B, Matuda SPT (2006) Biosynthetic diversity in plant triterpene cyclization ''Curr Opin Plant Biol'' 9:305-314 | * Philips DR, Rasbery JM, Bartel B, Matuda SPT (2006) Biosynthetic diversity in plant triterpene cyclization ''Curr Opin Plant Biol'' 9:305-314 | ||
| + | |} | ||
| − | ==={{Bilingual|Squalene Cyclase in Bacteria and Ferns | + | ==={{Bilingual|バクテリア・シダ類のスクアレン環化酵素|Squalene Cyclase in Bacteria and Ferns}}=== |
{{Twocolumn| | {{Twocolumn| | ||
| Line 259: | Line 271: | ||
{| style="text-align:center" | {| style="text-align:center" | ||
| − | | squalene<br/>[[Image:squalene.png]] | + | | squalene<br/>[[Image:squalene.png|150px]] |
| [[Image:Arrow00r.png]]<br/>squalene-hopene cyclase<ref>SH cyclase is the most investigated enzyme among squalene cyclases. <br/>''Ref.'' Kannenberg EL, Poralla K (1999) Hopanoid biosyntehsis and function in bacteria. ''Naturwissenschaften'' 86:168-76.</ref> | | [[Image:Arrow00r.png]]<br/>squalene-hopene cyclase<ref>SH cyclase is the most investigated enzyme among squalene cyclases. <br/>''Ref.'' Kannenberg EL, Poralla K (1999) Hopanoid biosyntehsis and function in bacteria. ''Naturwissenschaften'' 86:168-76.</ref> | ||
| 17α-deoxydammarenyl cation<ref>The C-17 chain of deoxydammarenyl cation is α-configuration, not β as in eukaryotes.<br/> | | 17α-deoxydammarenyl cation<ref>The C-17 chain of deoxydammarenyl cation is α-configuration, not β as in eukaryotes.<br/> | ||
| − | ''Ref.'' Wendt KU, Schulz GE, Corey EJ, Liu DR (2000) Enzyme mechanisms for polycyclic triterpene formation. [http://www3.interscience.wiley.com/journal/72515653/abstract?CRETRY=1&SRETRY=0 ''Angew Chem, Int Ed'' 39:2812-33]</ref> <br/>[[Image:Deoxydammarenyl cation.png]] | + | ''Ref.'' Wendt KU, Schulz GE, Corey EJ, Liu DR (2000) Enzyme mechanisms for polycyclic triterpene formation. [http://www3.interscience.wiley.com/journal/72515653/abstract?CRETRY=1&SRETRY=0 ''Angew Chem, Int Ed'' 39:2812-33]</ref> <br/>[[Image:Deoxydammarenyl cation.png|150px]] |
|- | |- | ||
| | | | ||
| Line 273: | Line 285: | ||
|} | |} | ||
|- | |- | ||
| − | |style="background-color:#ddf"| hopene<br/>[[Image:Hopene.png]] | + | |style="background-color:#ddf"| hopene<br/>[[Image:Hopene.png|150px]] |
| [[Image:Arrow00l.png]] | | [[Image:Arrow00l.png]] | ||
| − | | hopyl cation <br/>[[Image:Hopyl cation.png]] | + | | hopyl cation (c-c-c-c)<br/>[[Image:Hopyl cation.png|150px]] |
|} | |} | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | Hopanoids are widespread: they are found in bacteria, ferns, and geological sediments. | + | Hopanoids are widespread: they are found in bacteria, ferns, and geological sediments. They are not found, however, in archaea or animals. |
| + | |||
| | | | ||
| − | + | ホパノイドはバクテリアだけでなく、シダ類や地下堆積物からも見つかっています。しかしアーキアや動物からは見つかっていません。 | |
}} | }} | ||
| Line 294: | Line 307: | ||
<references/> | <references/> | ||
| − | ==={{Bilingual|Bis-oxidosqualene Cyclase | + | ==={{Bilingual|ビスオキシドスクアレン環化酵素|Bis-oxidosqualene Cyclase}}=== |
{{Twocolumn| | {{Twocolumn| | ||
Squalene is oxidized by squalene oxidase to become 2,3-oxidosqualene. | Squalene is oxidized by squalene oxidase to become 2,3-oxidosqualene. | ||
| Line 317: | Line 330: | ||
<references/> | <references/> | ||
| − | =={{Bilingual|Design of Tri-terpene ID numbers | + | =={{Bilingual|ID番号の設計|Design of Tri-terpene ID numbers}}== |
<center> | <center> | ||
12-DIGIT | 12-DIGIT | ||
Latest revision as of 11:55, 8 December 2012
Contents |
[edit] Triterpene (C30)
[edit] Ring configuration
[edit] Steroid
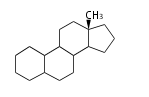
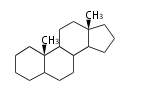
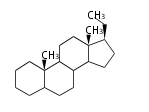
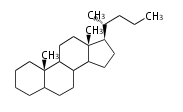
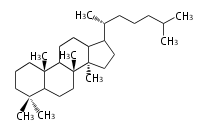
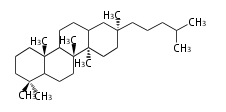
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. The rings B/C are always trans in all natural steroids. If the rings C/D are trans, it is called gonane. If its stereochemistry is unspecified, it is called sterane. Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are cis.
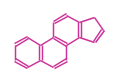 |
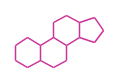
|
| Cyclopenta[a]phenanthrene | Gonane |
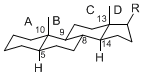
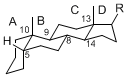
The majority of steroids have methyl groups sticking out from the bridgehead positions C-10 and C-13. When these methyl groups (or hydrogens) stand above the plane, they are called β-configuration. Those below the plane are called α-configuration. If the configuration at any site is unknown, it is indicated as ξ (Greek Xi). By default, hydrogen atoms or substituents at the positions C-8, 9, 10, 13, and 14 are assumed to be 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are.
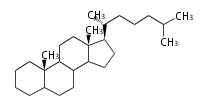 |
 |
|
| cholestane backbone | 5α-configuration | 5β-configuration |
[edit] Triterpenes
In almost all pentacyclic triterpenes in angiosperms, the methyl group at the DE-ring fusion is β-configuration. Some triterpenes in ferns, mosses, gymnosperms have α-methyl group at the DE-ring fusion.
[edit] Biosynthesis
[edit] Overview
The starting point is squalene, which is formed by joining two FPPs tail-to-tail. Bacterial cyclases use squalene directly[1], but those of the other species use 2,3-oxidosqualene for cyclization.
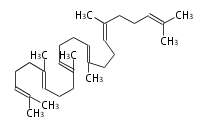
|
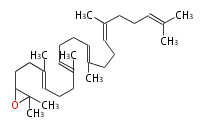
|
| squalene | 2,3-oxidosqualene |
- In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes.
- In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol (animals and fungi), cycloartenol (plants) or parkeol (sea cucumbers) by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts).
- In plants, a trace amount of phytosterols comes from lanosterol [2] At3g45130 is lanosterol synthase in Arabidopsis and its orthologs exist in asterids Taraxacum officinale and Panax ginseng and eurosid Luffa cylindrica. Lanosterol synthase exists broadly among eudicots [3]. Parkeol is also widespread in plants.
- In plants, various triterpenes arise from the 17β-dammarenyl cation.
- References
- ↑ Bacterial squalene cyclases can accept oxidosqualene as their substrates, but oxidosqualene usually does not exist in bacteria
- ↑ Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T (2009) Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis Proc Natl Acad Sci USA 106(3):725-730
- ↑ Kolesnikova MD, Xiong Q, Lodeiro S, Hua L, Matsuda SPT (2006) Lanosterol biosynthesis in plants Arch Biochem Biophys 447:87-95
[edit] Oxidosqualene Cyclase in Eukaryotes
Any path of reactions from the root (2,3-oxidosqualene) to any triterpene backbone with a colored background is catalyzed by a single enzyme called oxidosqualene cyclase (OSC) or terpene synthase h (tpsh).[1]
| Backbone Color Code: | Animals, fungi, and yeast | Plants only | |
|---|---|---|---|
| Six-membered rings: | chair (C), or boat (B) | ||
| 2,3-oxidosqualene | |||||||||||||||||

| |||||||||||||||||
|
| ||||||||||||||||
17β-protosteryl cation (C-B-C)[5] 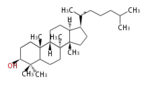
|
1,2-shift |
lanosteryl cation (C-B-C)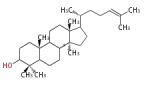
|
17β-dammarenyl cation (C-C-C)[6] 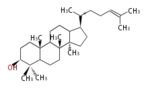
| ||||||||||||||
|
|
| |||||||||||||||
cation with the chain at C18 or C17 position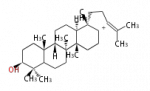 or or 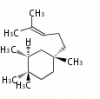
|
|
|
baccarenyl cation (C-C-C-C)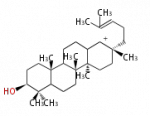
| ||||||||||||||
|
|
|
| ||||||||||||||
arborinyl cation (C-B-C-C)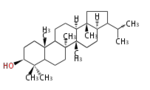
|
unnamed cation (C-B-C-C)
|
21α-hopyl cation (C-C-C-C) 21β-moretyl cation (C-C-C-C)[10] 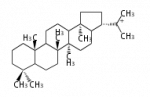
|
H18α-lupyl cation (C-C-C-C) H18β-lupyl cation (C-C-C-B) 
| ||||||||||||||
|
|
|
| ||||||||||||||
| arborinane (C-B-C-C) stictane (C-B-C-C-C)[11] |
hancokinane (C-B-C-C) | hopane (C-C-C-C) gammacerane (C-C-C-C-C) fernane (C-C-C-C) swertane (C-C-C-C-C) |
oleanane[12] (C-C-C-C-C) lupane (C-C-C-C) germanicane taraxastane (C-C-C-C-C) ursane (C-C-C-C-C/B) friedomadeirane (C-C-C-C)[13] | ||||||||||||||
| References |
|---|
|
| Reviews |
|
[edit] Squalene Cyclase in Bacteria and Ferns
Squalene cyclase (SC) or terpene synthase g (tpsg) are found in prokaryotes, ciliates, and lower plants (mosses and ferns) and can convert squalene, which is symmetric, as well as 2,3-oxidosqualene. Main products are hopanol and tetrahymanol, and only generate all-chair cations.
squalene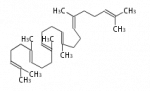
|
squalene-hopene cyclase[1] |
17α-deoxydammarenyl cation[2] 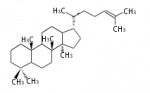
| |||
| |||||
hopene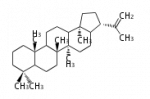
|
|
hopyl cation (c-c-c-c)
|
Hopanoids are widespread: they are found in bacteria, ferns, and geological sediments. They are not found, however, in archaea or animals.
Almost all mono-, di-, tri- and tetracyclic terpenes from squalene are found in ferns: Polypodium, Lemmaphyllum, and Pyrrosia. Notable exception is Z-Dammara-17(20),24-diene from moss Floribundaria[3]. Pentacyclic terpenes from squalene, on the other hand, are found also in angiosperms such as Achillea, Erysimum, Castanopsis and in Ascomycota.
- ↑ SH cyclase is the most investigated enzyme among squalene cyclases.
Ref. Kannenberg EL, Poralla K (1999) Hopanoid biosyntehsis and function in bacteria. Naturwissenschaften 86:168-76. - ↑ The C-17 chain of deoxydammarenyl cation is α-configuration, not β as in eukaryotes.
Ref. Wendt KU, Schulz GE, Corey EJ, Liu DR (2000) Enzyme mechanisms for polycyclic triterpene formation. Angew Chem, Int Ed 39:2812-33 - ↑ Toyota M, Masuda K, Asakawa Y (1998) Triterpenoid constituents of the moss Floribundaria aurea subsp. nipponica. Phytochemistry 48:297–299
[edit] Bis-oxidosqualene Cyclase
Squalene is oxidized by squalene oxidase to become 2,3-oxidosqualene. Further epoxidation of this symmetric molecule produces 2,3-(S)-22,23-(S)-bis-oxidosqualene, which is converted to 24,25-epoxylanostan-3-ol or 24,25-epoxycycloartan-3-ol. Epoxylanosterol is known to negatively regulate sterol biosynthesis[1].

|

|
| 2,3-oxidosqualene | 2,3-22,23-bis-oxidosqualene |
- ↑ Gardner RG, Shan H, Matsuda SPT, Hampton RY (2001) A positive oxysterol-derived signal for 3-hydroxy-3-methylglutaryl CoA reductase degradation in yeast. J Biol Chem 276:8681–869
[edit] Design of Tri-terpene ID numbers
12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
- y ... backbone structure (母核構造)
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
This category currently contains no pages or media.