Category:FL7
| Line 2: | Line 2: | ||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| + | |||
| + | {| class="wikitable" border="1" cellspacing="0" | ||
| + | |- | ||
| + | ! colspan="4"|2nd Class | ||
| + | |- | ||
| + | |FL7A||[[:Category:FL7A|Anthocyanin]]<br>[[Image:Fl7a.png|90px]] | ||
| + | |FL7D||[[:Category:FL7D|3-Deoxyanthocyanin]]<br>[[Image:Fl7d.png|90px]] | ||
| + | |} | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | The Greek word origin of anthocyanin is "flower" ἀνθός (anthos) and "blue" κυανός (kyanos). | + | The Greek word origin of anthocyanin is "flower" ἀνθός (anthos) and "blue" κυανός (kyanos). It was first used by Marquart (1835) to describe water-soluble pigments in red, blue, and purple from flowers. Nowadays, such water-soluble pigments from flowers and fruits are called anthocyan. The flavonoid backbone without sugar (shown below) is called anthocyanidin (it is called aglycon because it is devoid of glyco moiety), and structure with sugars is called anthocyanin. Except for carotenoid (yellow) or indigo (deep blue), most pigments of plant origin are anthocyanins. |
[[en:Anthocyanin]] | [[en:Anthocyanin]] | ||
| | | | ||
| − | アントシアニンの語源はギリシャ語で「花」意味するἀνθός (anthos)と, 「青」を意味するκυανός (kyanos) | + | アントシアニンの語源はギリシャ語で「花」意味するἀνθός (anthos)と, 「青」を意味するκυανός (kyanos)です。 Marquart (1835)が花の赤、紫、青に対応する水溶性色素の意味で使い始めました。今日、そうした水溶性色素はアントシアンと呼ばれます。そのうち、糖を持たず右上に示されたフラボノイド骨格だけの場合をアントシアニディンanthocyanidin (糖が無い形なのでa + glyco アグリコンと呼ばれます), 配糖体のものをアントシアニンanthocyaninと呼びます。 |
黄色のカロテノイドや藍色のインディゴを除くと、ほとんどの植物由来の色素はアントシアニンです。 | 黄色のカロテノイドや藍色のインディゴを除くと、ほとんどの植物由来の色素はアントシアニンです。 | ||
}} | }} | ||
| + | References | ||
| + | * Marquart LC. "Die Farben der Bluethen. Eine chemisch-physiol." Abhandlung, Bonn (Cited by Onslow MW. "The Anthocyanin Pigments of Plants," Cambridge University Press, 1925). | ||
===Target of fruit and flower color engineering=== | ===Target of fruit and flower color engineering=== | ||
| Line 19: | Line 29: | ||
アントシアニンは芳香環を3つ有し, 3-OH位置への糖転移は芳香環を安定させるために必要といわれています。 色は一般的には酸性で赤、アルカリ性で青ですが、[[Species:Viola|パンジー]]に様々な色(黄, オレンジ, 紫, 青, 濃紺など)があるように, 金属イオンや他の修飾基など様々な要素に左右されます。 | アントシアニンは芳香環を3つ有し, 3-OH位置への糖転移は芳香環を安定させるために必要といわれています。 色は一般的には酸性で赤、アルカリ性で青ですが、[[Species:Viola|パンジー]]に様々な色(黄, オレンジ, 紫, 青, 濃紺など)があるように, 金属イオンや他の修飾基など様々な要素に左右されます。 | ||
}} | }} | ||
| − | + | References | |
* Kroon J. et al. ''Plant J.'' 1994 Jan;5(1):69-80 PMID 8130799 | * Kroon J. et al. ''Plant J.'' 1994 Jan;5(1):69-80 PMID 8130799 | ||
* Boss PK. et al. ''Plant Mol Biol.'' 1996 Nov;32(3):565-9 PMID 8980508 | * Boss PK. et al. ''Plant Mol Biol.'' 1996 Nov;32(3):565-9 PMID 8980508 | ||
* Fukuchi-Mizutani M. et al. ''Plant Physiol.'' 2003 Jul;132(3):1652-63 PMID 12857844 (research paper on Blue Rose) | * Fukuchi-Mizutani M. et al. ''Plant Physiol.'' 2003 Jul;132(3):1652-63 PMID 12857844 (research paper on Blue Rose) | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Database statistics データベース統計==<!--- see also FL1---> | ==Database statistics データベース統計==<!--- see also FL1---> | ||
Revision as of 21:06, 10 October 2008
Anthocyanin
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid
| 2nd Class | |||
|---|---|---|---|
| FL7A | Anthocyanin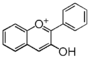
|
FL7D | 3-Deoxyanthocyanin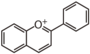
|
The Greek word origin of anthocyanin is "flower" ἀνθός (anthos) and "blue" κυανός (kyanos). It was first used by Marquart (1835) to describe water-soluble pigments in red, blue, and purple from flowers. Nowadays, such water-soluble pigments from flowers and fruits are called anthocyan. The flavonoid backbone without sugar (shown below) is called anthocyanidin (it is called aglycon because it is devoid of glyco moiety), and structure with sugars is called anthocyanin. Except for carotenoid (yellow) or indigo (deep blue), most pigments of plant origin are anthocyanins.
References
- Marquart LC. "Die Farben der Bluethen. Eine chemisch-physiol." Abhandlung, Bonn (Cited by Onslow MW. "The Anthocyanin Pigments of Plants," Cambridge University Press, 1925).
Target of fruit and flower color engineering
Anthocyanin contains 3 aromatic rings, and glycosylation at the 3-OH position is necessary for stabilizing the aromatic ring. The general color of anchocyanin is red in acidic environment and purple/blue in alkali. The colors are, however, dependent on many other factors such as additional modifications and metal ions as is suggested by multi-color flowers such as pansy (yellow, orange, purple, violet, deep blue).
References
- Kroon J. et al. Plant J. 1994 Jan;5(1):69-80 PMID 8130799
- Boss PK. et al. Plant Mol Biol. 1996 Nov;32(3):565-9 PMID 8980508
- Fukuchi-Mizutani M. et al. Plant Physiol. 2003 Jul;132(3):1652-63 PMID 12857844 (research paper on Blue Rose)
Database statistics データベース統計