Category:PK
m |
m |
||
| Line 1: | Line 1: | ||
| + | {{PKS/Header}} | ||
| + | |||
{{Huge|Polyketide (ポリケチド)}} | {{Huge|Polyketide (ポリケチド)}} | ||
{| style="float:right" | {| style="float:right" | ||
Revision as of 19:23, 18 December 2010
| Polyketide Top | Species List | UniRef90 Class | UniRef50 Class | Gene Class | Domains (by CDD) |
Domains (by MAPSI) |
Polyketide (ポリケチド)
|
Class Overview
Polyketides are synthesized through the polymerization of acetyl units (β-ketomethylene) as in fatty acid biosynthesis. Typical starter units are short-chain fatty acids (e.g. acetyl-CoA or propionyl-CoA), on to which extender units (e.g. malonyl-CoA or methylmalonyl-CoA) are repeatedly polymerized. The key reactions for the chain extension are:
- Claisen condensation by β-ketoacyl synthase (KS)
- an acyltransferase (AT), and
- an acyl carrier protein (ACP).
After elongation, β-ketone is reduced. In fatty acid biosynthesis, the chain is fully reduced by the following three steps:
- Reduction to an alcohol by ketoreductase (KR),
- Dehydration to the conjugated ester by dehydratase (DH), and
- Reduction of the double bond by enoyl reductase (ER).
Finally, the chain is terminated by a thioesterase (TE) activity and allows Claisen cyclization (CYC).
| 1st Class | ||
|---|---|---|
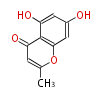
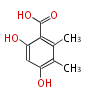
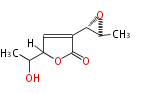
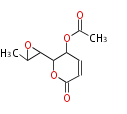
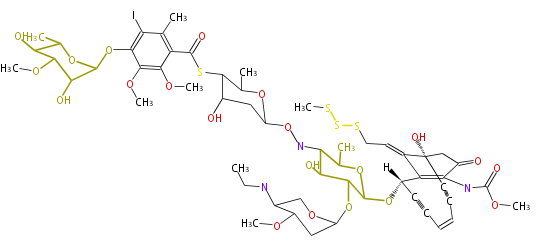
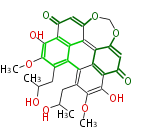
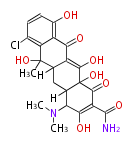
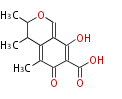
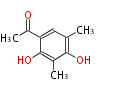
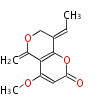
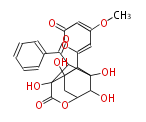
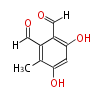
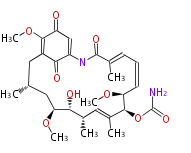
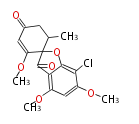
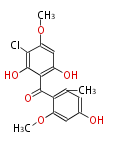
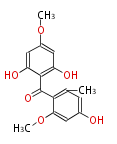
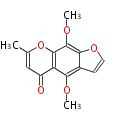
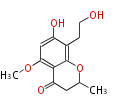
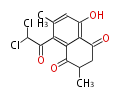
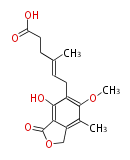
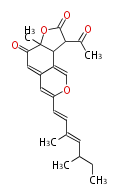
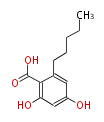
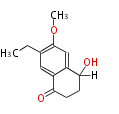
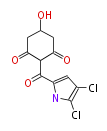
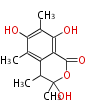
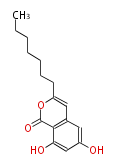
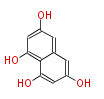
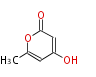
| PK4: Four C2 Units orsellinic acid, 6-methylsalicylic acid, triacetic acid lactone, asperlin, usnic acid, methylphloracetophenone, penicillic acid, patulin |
PK5: Five C2 Units citrinin, aflatoxin, augenone, sepedonin, stipitatonic acid |
PK6: Six C2 Units plumbagin, 7-methyljuglone, juglone, variotin |
| PK7: Seven and eight C2 Units Anthraquinone rings |
PK9: Nine C2 Units Tetracyclines | |
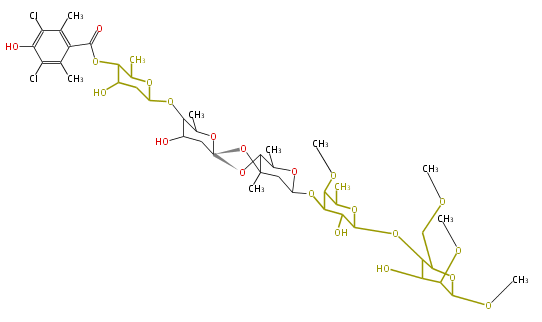
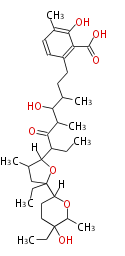
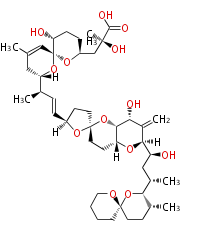
| Linear Chain and Related (L) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
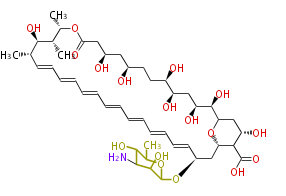
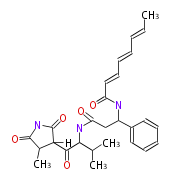
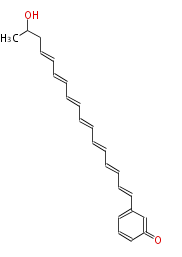
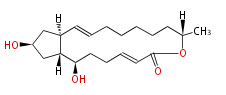
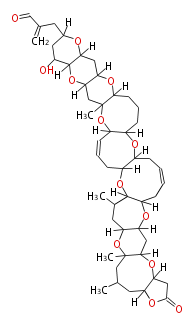
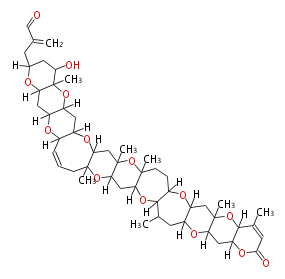
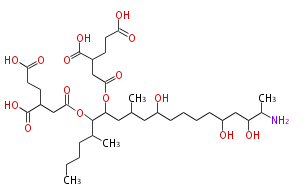
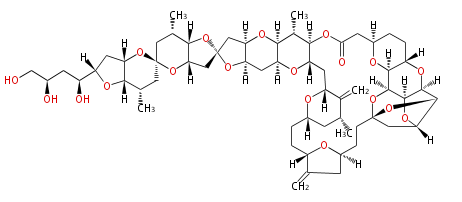
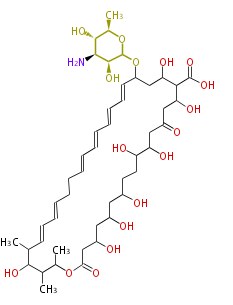
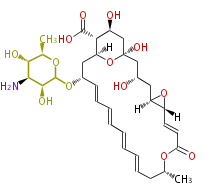
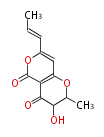
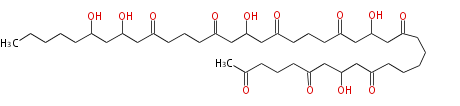
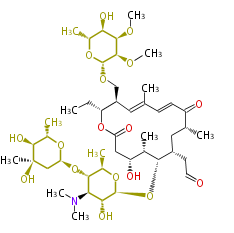
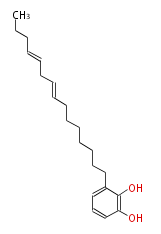
Acetogenins (LA) | ||||||||||||
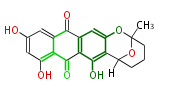
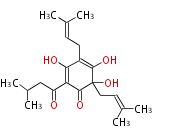
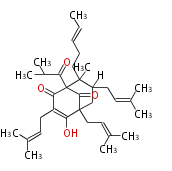
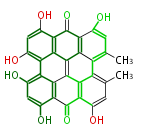
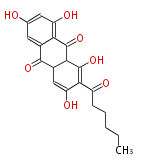
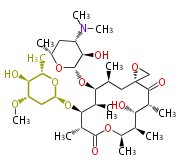
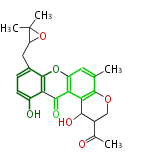
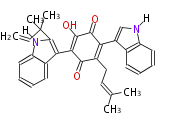
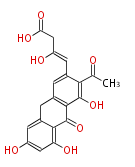
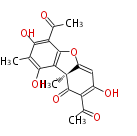
| Aromatic and Diels-Alder Related (most often by iterative type II) | ||||||||||||||
|
|
| ||||||||||||
|
| |||||||||||||
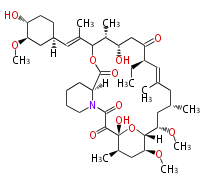
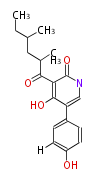
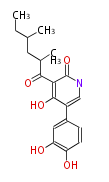
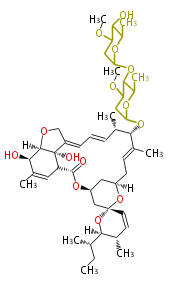
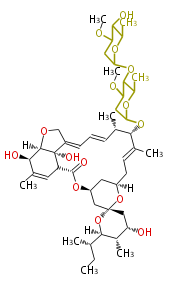
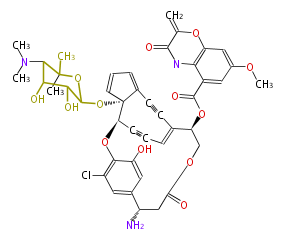
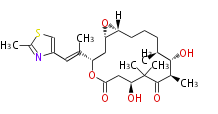
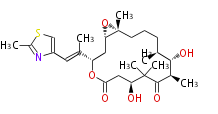
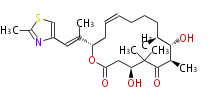
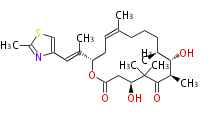
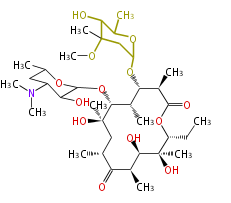
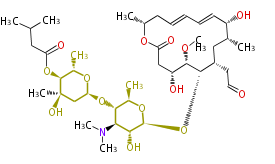
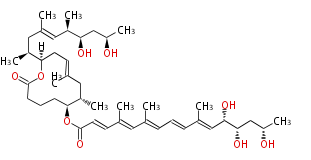
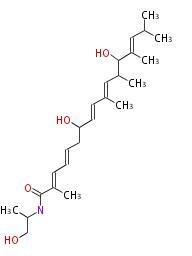
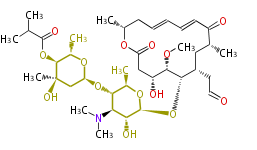
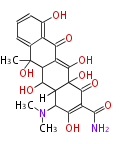
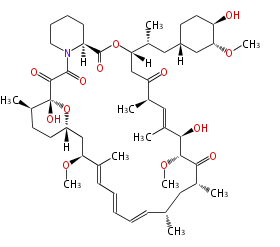
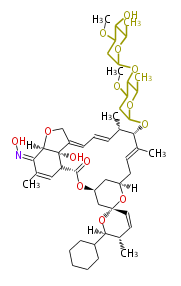
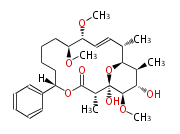
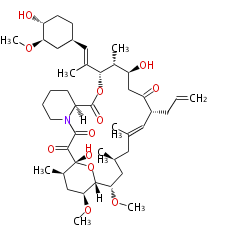
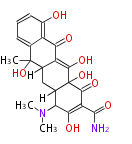
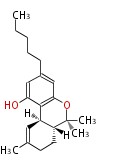
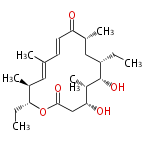
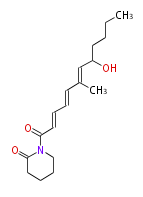
| Macrolides (most often by non-iterative type I) | ||||||||||||||
|
|
| ||||||||||||
|
|
| ||||||||||||
|
| |||||||||||||
Polyketide Synthase (PKS)
| species | Actinomycetes | Cyanobacteria | γ-Proteobacteria | Fungi | Dinoflagellates |
|---|---|---|---|---|---|
| Type-I PKS | Ο | Ο | Ο | Χ | Ο |
| Type-II PKS | Ο | Χ | Χ | Ο | Χ |
| NRPS | Ο | Ο | Ο | Ο | Χ |
| deoxysugar | Ο | Χ | Χ | Χ | Χ |
| Terpene | Δ | Χ | Χ | Ο | Χ |
Type I PKS (non-iterative)
- Multi catalytic domains exist in a single protein
- Chain length is determined by the number of catalytic domains.
- Products are non-aromatic and have larger masses.
Ref. Erythromycin biosynthesis in Nat Prod Rep 18, 380 (2001)
Type II PKS (iterative)
- Three proteins (KSα, KSβ, ACP) are repeatedly used for carbon chain elongation.
- Chain length is determined by another protein, CLF.
- In bacteria, products are aromatic (e.g. chiorotetracycline, pradimicin).
- In fungi, products are both non-aromatic and aromatic.
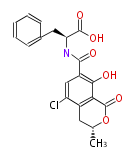
Non-ribosomal peptide synthase (NRPS)
Coupling with PKS and NRPS
- vancomycin ()
- leinamycin (Curr opin chem biol 7:285, 2003)
- pseurotin (chem bio chem 8:1736-1743, 2007)
- curacin (curr opin chem biol 13:216, 2009)
- epothilone
- rapamycin
PKS in Fungi
- both aromatic and non-aromatic compounds are generated by iterative PKS
- methyl branch is transferred from methionine, not methylmalonyl CoA
Ref. Dewick, PM Medicinal Natural Products (2009)
Decoration
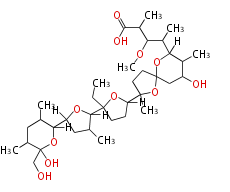
deoxysugars
deoxygenation, c-methylation, amination, n-methylation, ketosugar,
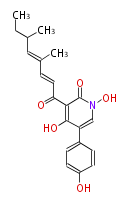
Unusual structures
| Phoma | zaragozic acid, phomoidoride | Streptomyces | yatakemycin, leinamycin, saframycin, neocarzinostatin, staurosporin, FR182877 | Other bacteria | PKS-NRPS hybrid type
Curacin A (Lyngbya), Shiphonazole (Herpetosiphon), Jamaicamide A (Lyngbya), Cylindrospermopsin (Cylindrospermopsis) |
|---|
Cite error:
<ref> tags exist, but no <references/> tag was found