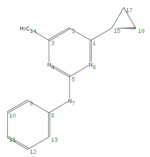
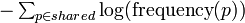MassBank:KOX00694p
From Metabolomics.JP
(Redirected from MassBank:KOX00694)
| General Index | Ion Frequency | Prec.-Product | Neutral Loss | Help |
| IDs and Links | |
|---|---|
| MassBank | Cyprodinil |
| CAS | 121552-61-2 |
| Keio ID | C172+ |
Contents |
Top 10 Similar Molecules of Cyprodinil
| Ranking | About scoring |
|---|---|
|
The similarity score between two molecules is defined as the sum of Shannon entropy of ionic formulas shared by the molecules:
|
Links
Annotations
| Precursor | Product | Comments |
|---|---|---|
| C14H16N3 (226) | C13H12N3 (210) | c |
| C14H16N3 (226) | C12H12N3 (198) | d |
| C14H16N3 (226) | C11H10N3 (184) | e |
| C14H16N3 (226) | C8H9N2 (133) | f |
| C14H16N3 (226) | C7H10N (108) | b[i] |
| C14H16N3 (226) | C6H8N (94) | j |
| C14H16N3 (226) | C6H7N (93) | j,rad. |
| C14H16N3 (226) | C7H7 (91) | k |
| C14H16N3 (226) | C6H5 (77) | a |
| C14H16N3 (226) | C5H5 (65) | h |
| C14H16N3 (226) | C4H3 (51) | g |
Precursor-Product Relationship
| About the PP Table (行列表示について) | |
|---|---|
| The matrix is viewed columnwise. The topmost precursor ion in bold face produces the product ions beneath it.
Each formula in matrix cells corresponds to the neutral loss. Blackout cells indicate products that cannot be derived, and orange cells indicate a structurally plausible link produced by cleaving a single chemical bond (in cases of ring-opening, two bonds). |
行列は列方向に見ます。最上段太字の前駆イオン(precursor ion)が直下の生成イオン群(product ions)になると解釈します。
行列要素に書かれている組成式はニュートラルロスです。黒は前駆イオンから生成しえない関係、オレンジは分子構造における共有結合1本の切断(開環の場合は2本)で生じる関係を意味します。 |
| KOX00694p | 226 C14H16N3 |
210 C13H12N3 |
198 C12H12N3 |
185 C12H13N2 |
184 C11H10N3 |
159 C10H11N2 |
144 C10H10N |
133 C8H9N2 |
118 C7H6N2 |
108 C7H10N |
94 C6H8N |
93 C6H7N |
91 C7H7 |
77 C6H5 |
65 C5H5 |
51 C4H3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 226 C14H16N3 |
| |||||||||||||||
| 210 C13H12N3 | CH4 |
| ||||||||||||||
| 198 C12H12N3 | C2H4 | C |
| |||||||||||||
| 185 C12H13N2 | C2H3N |
| ||||||||||||||
| 184 C11H10N3 | C3H6 | C2H2 | CH2 |
| ||||||||||||
| 159 C10H11N2 | C4H5N | C3HN | C2HN | C2H2 |
| |||||||||||
| 144 C10H10N | C4H6N2 | C3H2N2 | C2H2N2 | C2H3N | CN2 | HN |
| |||||||||
| 133 C8H9N2 | C6H7N | C5H3N | C4H3N | C4H4 | C3HN | C2H2 |
| |||||||||
| 118 C7H6N2 | C7H10N | C6H6N | C5H6N | C5H7 | C4H4N | C3H5 | CH3 |
| ||||||||
| 108 C7H10N | C7H6N2 | C6H2N2 | C5H2N2 | C5H3N | C4N2 | C3HN | C3 |
| ||||||||
| 94 C6H8N | C8H8N2 | C7H4N2 | C6H4N2 | C6H5N | C5H2N2 | C4H3N | C4H2 | C2HN | CH2 |
| ||||||
| 93 C6H7N | C8H9N2 | C7H5N2 | C6H5N2 | C6H6N | C5H3N2 | C4H4N | C4H3 | C2H2N | CH3 | H |
| |||||
| 91 C7H7 | C7H9N3 | C6H5N3 | C5H5N3 | C5H6N2 | C4H3N3 | C3H4N2 | C3H3N | CH2N2 | H3N |
| ||||||
| 77 C6H5 | C8H11N3 | C7H7N3 | C6H7N3 | C6H8N2 | C5H5N3 | C4H6N2 | C4H5N | C2H4N2 | CHN2 | CH5N | H3N | H2N | CH2 |
| ||
| 65 C5H5 | C9H11N3 | C8H7N3 | C7H7N3 | C7H8N2 | C6H5N3 | C5H6N2 | C5H5N | C3H4N2 | C2HN2 | C2H5N | CH3N | CH2N | C2H2 | C |
| |
| 51 C4H3 | C10H13N3 | C9H9N3 | C8H9N3 | C8H10N2 | C7H7N3 | C6H8N2 | C6H7N | C4H6N2 | C3H3N2 | C3H7N | C2H5N | C2H4N | C3H4 | C2H2 | CH2 |
|