Licoricesaponin A3
From Metabolomics.JP
(Difference between revisions)
| Line 47: | Line 47: | ||
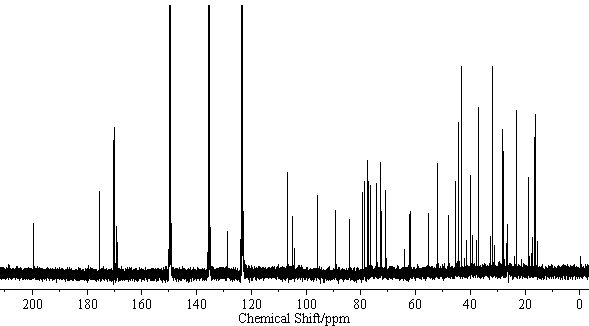
[[Image:Licoricesaponin_A3_CNMR.gif|thumb|600px|none|<sup>13</sup>C-NMR Licoricesaponin A3 Methyl ester / pyridine-<i>d</i><sub>5</sub>]] | [[Image:Licoricesaponin_A3_CNMR.gif|thumb|600px|none|<sup>13</sup>C-NMR Licoricesaponin A3 Methyl ester / pyridine-<i>d</i><sub>5</sub>]] | ||
| + | |||
| + | |||
| + | ==Spectroscopic Data== | ||
| + | |||
| + | {| class="wikitable" style="width:80%" | ||
| + | |||
| + | '''Free acid''' | ||
| + | |- | ||
| + | | '''M.P.''' || 196 - 199 °C | ||
| + | |- | ||
| + | | '''IR''' (KBr)|| 1716 (ester), 1741, 1650 (alpha beta-unsaturation ketone) cm<sup>-1</sup> | ||
| + | |} | ||
| + | {| class="wikitable" style="width:80%" | ||
| + | '''Dimethyl ester''' | ||
| + | |- | ||
| + | | '''M.P.''' || 205 - 208 °C | ||
| + | |- | ||
| + | | '''IR''' (KBr)|| 3420, 1740, 1650 cm<sup>-1</sup> | ||
| + | |- | ||
| + | | '''<sup>13</sup>C-NMR''' (C<sub>5</sub>D<sub>5</sub>N, 22.5MHz)|| C-3) 89.0, (11) 199.3, (12) 128.2, (13) 169.1, (18) 47.9, (22) 39.4, (30) 175.4 '''GlcUA I''' (1) 106.1, (2) 75.8, (3) 76.0, (4) 72.2, (5) 77.0, (6) 169.6 '''GlcUA II''' (1)106.1, (2) 75.8, (3) 77.0, (4) 72.3, (5) 77.0, (6) 169.7 '''Glc''' (1) 95.4, (2) 73.6, (3) 78.7, (4) 70.7, (5)78.1, (6) 61.8 | ||
| + | |} | ||
| + | <small>M. Yoshikawa et al., Chem.Pharm.Bull., 36, 3710 (1988).</small> | ||
Latest revision as of 11:29, 9 February 2010
Upper classes
| IDs and Links | |
|---|---|
| LipidBank | [1] |
| LipidMaps | [2] |
| CAS | 118325-22-7 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | |
| CDX file | |
| MOL file | Licoricesaponin A3.mol |
| Licoricesaponin A3 | |
|---|---|
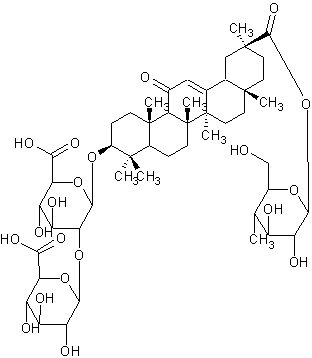
| |
| Structural Information | |
| Systematic Name | (3.beta.,20.beta.)-29-(.beta.-D-glucopyranosyloxy)-11,29-dioxoolean-12-en-3-yl 2-O-.beta.-D-glucopyranuronosyl-.beta.-D-Glucopyranosiduronic acid |
| Common Name |
|
| Symbol | |
| Formula | C48H72O21 |
| Exact Mass | 984.456609366 |
| Average Mass | 985.07268 |
| SMILES | C(C86)(=C1)C(CCC(CCC(C)(C8)C(OC(O7)C(C(C(O)C7CO)O) |
| Physicochemical Information | |
| Melting Point | |
| Boiling Point | |
| Density | |
| Optical Rotation | |
| Reflactive Index | |
| Solubility | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Chromatograms | |
Contents |
[edit] Mass Spectral Data
| Instrument | Shimadzu LC-IT-TOF MS ESI |
| Column | Waters Atlantis T3 (2.1 mm x 150 mm) |
| Column Temperature | 40 °C |
| Solvent A | 5 mM Ammonium acetate solution |
| Solvent B | acetonitrile |
| Gradient | 10% to 100% Solvent B (0-30 min), 100% Solvent B (30-40 min) |
| Source voltage | – 3.5 kV (negative), 4.5 kV (positive) |
| Capillary temperature | 200 °C |
| Nebulizer gas | 1.5 l/min |
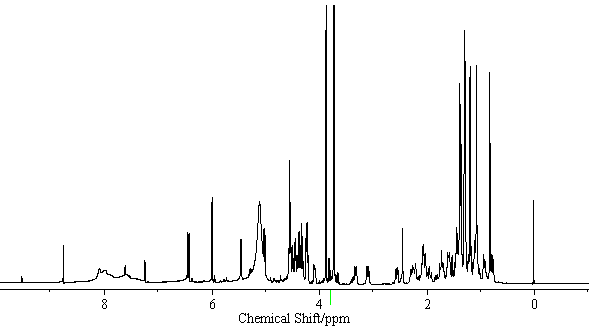
[edit] NMR Data
[edit] Spectroscopic Data
| M.P. | 196 - 199 °C |
| IR (KBr) | 1716 (ester), 1741, 1650 (alpha beta-unsaturation ketone) cm-1 |
| M.P. | 205 - 208 °C |
| IR (KBr) | 3420, 1740, 1650 cm-1 |
| 13C-NMR (C5D5N, 22.5MHz) | C-3) 89.0, (11) 199.3, (12) 128.2, (13) 169.1, (18) 47.9, (22) 39.4, (30) 175.4 GlcUA I (1) 106.1, (2) 75.8, (3) 76.0, (4) 72.2, (5) 77.0, (6) 169.6 GlcUA II (1)106.1, (2) 75.8, (3) 77.0, (4) 72.3, (5) 77.0, (6) 169.7 Glc (1) 95.4, (2) 73.6, (3) 78.7, (4) 70.7, (5)78.1, (6) 61.8 |
M. Yoshikawa et al., Chem.Pharm.Bull., 36, 3710 (1988).