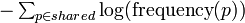MassBank:KOX00856p
From Metabolomics.JP
Revision as of 13:43, 4 November 2009 by Move page script (Talk)
| General Index | Ion Frequency | Prec.-Product | Neutral Loss | Help |
| IDs and Links | |
|---|---|
| MassBank | Propranolol |
| CAS | 525-66-6 |
| Keio ID | P192+ |
Contents |
Top 10 Similar Molecules of Propranolol
| Ranking | About scoring |
|---|---|
|
The similarity score between two molecules is defined as the sum of Shannon entropy of ionic formulas shared by the molecules:
|
Links
Annotations
| Precursor | Product | Comments |
|---|---|---|
| C16H22NO2 (260) | C11H9O (157) | f |
| C16H22NO2 (260) | C10H9O (145) | g |
| C16H22NO2 (260) | C10H7 (127) | h |
| C16H22NO2 (260) | C6H14NO (116) | i,-H2O |
| C16H22NO2 (260) | C4H10N (72) | b |
| C13H11O (183) | C13H9 (165) | IT |
| C13H11O (183) | C12H11 (155) | IT |
| C6H14NO (116) | C6H12N (98) | IT |
| C6H14NO (116) | C5H12N (86) | IT |
| C6H14NO (116) | C3H8NO (74) | IT |
| C6H14NO (116) | C4H10N (72) | IT |
Precursor-Product Relationship
| About the PP Table (行列表示について) | |
|---|---|
| The matrix is viewed columnwise. The topmost precursor ion in bold face produces the product ions beneath it.
Each formula in matrix cells corresponds to the neutral loss. Blackout cells indicate products that cannot be derived, and orange cells indicate a structurally plausible link produced by cleaving a single chemical bond (in cases of ring-opening, two bonds). |
行列は列方向に見ます。最上段太字の前駆イオン(precursor ion)が直下の生成イオン群(product ions)になると解釈します。
行列要素に書かれている組成式はニュートラルロスです。黒は前駆イオンから生成しえない関係、オレンジは分子構造における共有結合1本の切断(開環の場合は2本)で生じる関係を意味します。 |
| KOX00856p | 260 C16H22NO2 |
183 C13H11O |
165 C13H9 |
157 C11H9O |
155 C12H11 |
145 C10H9O |
141 C11H9 |
127 C10H7 |
116 C6H14NO |
98 C6H12N |
86 C5H12N |
74 C3H8NO |
72 C4H10N |
58 C3H8N |
56 C3H6N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 260 C16H22NO2 |
| ||||||||||||||
| 183 C13H11O | C3H11NO |
| |||||||||||||
| 165 C13H9 | C3H13NO2 | H2O |
| ||||||||||||
| 157 C11H9O | C5H13NO | C2H2 |
| ||||||||||||
| 155 C12H11 | C4H11NO2 | CO |
| ||||||||||||
| 145 C10H9O | C6H13NO | C3H2 | C |
| |||||||||||
| 141 C11H9 | C5H13NO2 | C2H2O | C2 | O | CH2 |
| |||||||||
| 127 C10H7 | C6H15NO2 | C3H4O | C3H2 | CH2O | C2H4 | H2O | CH2 |
| |||||||
| 116 C6H14NO | C10H8O |
| |||||||||||||
| 98 C6H12N | C10H10O2 | H2O |
| ||||||||||||
| 86 C5H12N | C11H10O2 | CH2O | C |
| |||||||||||
| 74 C3H8NO | C13H14O | C3H6 |
| ||||||||||||
| 72 C4H10N | C12H12O2 | C2H4O | C2H2 | CH2 |
| ||||||||||
| 58 C3H8N | C13H14O2 | C3H6O | C3H4 | C2H4 | O | CH2 |
| ||||||||
| 56 C3H6N | C13H16O2 | C3H8O | C3H6 | C2H6 | H2O | CH4 | H2 |
|