Category:FL7
Anthocyanidin, Anthocyanin
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid
| 2nd Class | |||
|---|---|---|---|
| FL7A | Anthocyani(di)n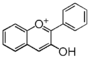
|
FL7D | 3-Deoxyanthocyani(di)n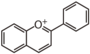
|
Contents |
Overview
Distribution
Anthocyanin is almost ubiquitous, whereas 3-Deoxyanthocyanin (luteolinidin and apigeninidin) is found only in some lower plants (moss and fern[1][2][3]) and in sorghum (Sorghum bicolor [4]), gloxinia (Sinningia cardinalis) and many species in Gesneriaceae[5].
The 9 known families without anthocyanin are Aizoaceae, Amaranthaceae, Basellaceae, Cactaceae, Chenopodiaceae, Didiereaceae, Nyctaginaceae, Phytolaccaceae, Portulacaceae (all in the order Caryophylalles. However, Caryophyllaceae and Molluginaceae synthesize anthocyanins). These families are considered to have unfunctional genes, and instead synthesize the betalain instead of anthocyanin.[6]
Word origin
The Greek word origin of anthocyanin is "flower" ἀνθός (anthos) and "blue" κυανός (kyanos). It was first used by Marquart to describe water-soluble pigments in red, blue, and purple from flowers [7]. Nowadays, such water-soluble pigments from flowers and fruits are called anthocyan. The flavonoid backbone without sugar (shown below) is called anthocyanidin (it is called aglycon because it is devoid of glyco moiety), and structure with sugars is called anthocyanin. Except for carotenoid (yellow) or indigo (deep blue), most pigments of plant origin are anthocyanins.
- ↑ Bendz G, Martensson O, Terenlus L: Moss pigments I. The anthocyanins of Bryum cryophilum O. Mart. Acta Chem Scand 1962 16:1183-1190
- ↑ Bendz G, Martensson O: Moss pigments II. The anthocyanins of Bryum rutilans Brid. and Bryum weigelii Spreng. Acta Chem Scand 1963 17:266
- ↑ Comparative biochemistry of the flavonoids-II. 3-Desoxyanthocyanins and their systematic distribution in ferns and gesnerads. Phytochemistry 1966 5:589-600
- ↑ Nip WK, Burns EE: Pigment characterization in grain Sorghum. Cereal Chem 1969 46:490-495 also in 1971 48:74-80
- ↑ Harborne JB: Comparative biochemistry of the flavonoids-II. 3-Desoxyanthocyanins and their systematic distribution in ferns and gesnerads. Phytochemistry 5:589-600
- ↑ Piattelli M, Minale L: Pigments of centrospermae-II. Distribution of betacyanins. Phytochemistry 1964 3:547-557
- ↑ Marquart LC. "Die Farben der Bluethen. Eine chemisch-physiol." Abhandlung, Bonn, 1835 (Cited by Onslow MW. "The Anthocyanin Pigments of Plants," Cambridge University Press, 1925).
Function
Fruit- and flower-colors
Anthocyanin contains 3 aromatic rings, and glycosylation at the 3-OH position is necessary for stabilizing the aromatic ring. The general color of anchocyanin is red in acidic environment and purple/blue in alkali. The colors are, however, dependent on many other factors such as additional modifications and metal ions as is suggested by multi-color flowers such as pansy (yellow, orange, purple, violet, deep blue). (BY Masanori Arita)
Phytoalexin
In sorghum, the 3-deoxyanthocyanidins are a class of phytoalexins, the class of compound to fend off fungi [1][2] They are produced within a cell under appressorial attack, first inside endoplasmic reticulum and then as colorless inclusions to the penetration site. The inclusions become yellow, and deep red with time. When the inclusions are broken by appressoria, the deoxyanthocyanidins kill the host cell and the pathogen together.
- ↑ Snyder BA, Nicholson RL (1990) "Synthesis of phytoalexins in sorhum as a site-specific response to fungal ingress" Science 248:1637-1639 PMID 17746504
- ↑ Nicholson RL, Wood KV (2001) "Phytoalexins and secondary products, where are they and how can we measure them?" Physiol Mol Plant Pathol 59:63-69
Biosynthesis
The six common anthocyanidins are the product of three different branches. In the figure below, horizontal shifts are hydroxylation and vertical, methylation.
| Biosynthesis (continued from the chart in Flavonoid) | ||||||
|---|---|---|---|---|---|---|
| (red or orange) | (blue or magenta) | (blue or purple) | ||||
| pelargonidin |
B-ring hydroxylation |
cyanidin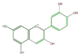
|
B-ring hydroxylation |
delphinidin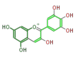
| ||
peonidin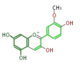
|
petunidin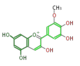
| |||||
|
||||||
malvidin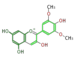
| ||||||
All three classes exist in most angio- and gymnosperm orders, and hydroxylating enzymes (F3'H and F3'5'H) show high sequence homology throughout orders. However, genetic changes cause genus-specific inactivation of these pathways.
| Genus (Family) | Inactivation | Cause |
|---|---|---|
| Arabidopsis (Brassicaceae) Petunia (Solanaceae) Cymbidium (Orchidaceae) |
pelargonidin | DFR does not metabolize dihydrokaempferol. |
| Chrysanthemum (Asteraceae) Dianthus (Caryophyllaceae) Ipomoea (Convolvulaceae) |
delphinidin | F3'5'H is absent. |
Structure
Most anthocyanins in autumn leaves are comparatively simple and 3-O-glycosilated (glucoside, galactoside, rhamnoside, gentiobiosid and rutinoside) and/or acylated (p-coumaric, caffeic, ferulic, gallic, acetyl, malonic, malic etc.). Those in storage organs often show complicated structures (see Ipomoea and Dioscorea).
Database statistics
Major Plant Families
|
|
The number in each family is counted as the number of genera (not species) listed in our registered references. Each reference record is accessible by clicking the link in compound pages. The taxonomy follows the APG-II classification. For details (or if the figure is broken), visit this page. 各科のカウントは種名でなく文献に記載された属名の数です。文献は代謝物ページのリンクからたどれ、分類はAPG-IIです。左の図が表示されない場合はここをクリックしてください。 |