Glycyrrhizin
From Metabolomics.JP
Upper classes
| IDs and Links | |
|---|---|
| LipidBank | [1] |
| LipidMaps | [2] |
| CAS | 1405-86-3 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | |
| CDX file | |
| MOL file | Glycyrrhizin.mol |
| Glycyrrhizin | |
|---|---|

| |
| Structural Information | |
| Systematic Name | (3beta,20beta)-20-Carboxy-11-oxo-30-norolean-12-en-3-yl 2-O-beta-D-glucopyranuronosyl-alpha-D-glucopyranosiduronic acid |
| Common Name |
|
| Symbol | |
| Formula | C42H62O16 |
| Exact Mass | 822.4037859360001 |
| Average Mass | 822.93208 |
| SMILES | C(C7(C)2)(=CC(C(C7(C)6)C(C5CC6)(CCC(C5(C)C)OC(C(OC |
| Physicochemical Information | |
| Melting Point | |
| Boiling Point | |
| Density | |
| Optical Rotation | |
| Reflactive Index | |
| Solubility | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Chromatograms | |
Contents |
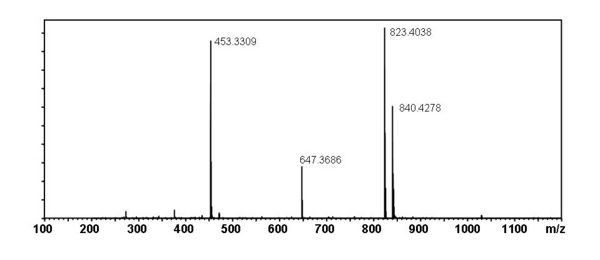
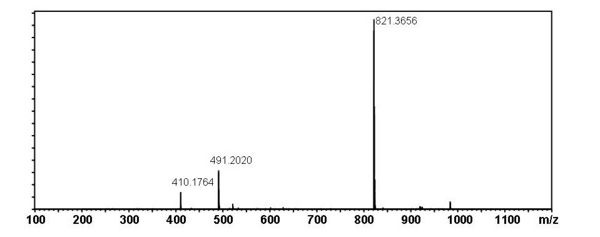
Mass Spectral Data
| Instrument | Shimadzu LC-IT-TOF MS ESI |
| Column | Waters Atlantis T3 (2.1 mm x 150 mm) |
| Column Temperature | 40 °C |
| Solvent A | 5 mM Ammonium acetate solution |
| Solvent B | acetonitrile |
| Gradient | 10% to 100% Solvent B (0-30 min), 100% Solvent B (30-40 min) |
| Source voltage | – 3.5 kV (negative), 4.5 kV (positive) |
| Capillary temperature | 200 °C |
| Nebulizer gas | 1.5 l/min |
Proposed Fragmentation Pathway
Spectroscopic Data
| M.P.1 | 217 °C (decomp.) |
| IR (KBr)1 | 3400, 1720, 1640 cm-1 |
| 13C-NMR (CDCl3, 22.5MHz)2 | C-1) 40.15, (2) 26.96, (3) 90.74, (4) 40.47, (5) 56.40, (6) 18.34, (7) 32.87, (8) 44.49, (9) 62.99, (10) 37.89, (11) 202.52, (12) 128.79, (13) 171.90, (14) 46.64, (15) 27.48, (16) 27.32, (17) 32.87, (18) 49.77, (19) 42.27, (20) 44.47, (21) 31.92, (22) 38.91, (23) 28.20, (24) 16.78, (25) 16.97, (26) 19.27, (27) 23.83, (28) 28.72, (29) 29.15, (30) 180.26 GlcUA I (1) 106.01, (2) 83.78, (3) 77.03, (4) 72.85, (5) 76.05, (6) 172.29 GlcUA II (1) 105.10, (2) 76.05, (3) 77.35, (4) 72.75, (5) 77.09, (6) 172.29 |
1) M.-T. Wang et al., Yaoxue Xuebao, 21, 510 (1986)., 2) G.A. Tolstikov et al., Khim.Prir.Soedin., 25, 500 (1989).